In the past, oral supplementation with the superoxide dismutase enzyme in order to boost the body’s antioxidant defenses has been ineffective. This is due to the biochemical conditions experienced in the gastrointestinal tract, leading to degradation of the enzyme and subsequently rendering it useless. This technical publication reviews the science related to GliSODin, a trade name for SOD-rich cantaloupe melon combined with wheat gliadin. Science is presented to demonstrate that combination with gliadin protects the SOD during passage through the stomach and enhances absorption once inside the intestine.
Superoxide dismutase (SOD) a main antioxidant defenses
Superoxide dismutase (SOD) constitutes part of the body’s front line in antioxidant defenses, helping to maintain the physiological oxidant-antioxidant balance. However, this balance can be disturbed by a number of different factors, including aging, smoking, pollution, exposure to sunlight, infection and the subsequent immune response, and high intensity exercise. Under such conditions the body experiences oxidative stress and this is linked with increased risk of chronic disease.
Antioxidant and imuno modulation properties
Results from in vitro, in vivo and human studies which show both the increase in antioxidant status as well as the reduction of markers of oxidative stress. It will also be shown that Glisodin’s bioactivity in humans has been demonstrated with a daily dose of as little as 250 mg over a period of just 14 days.
Anti-inflammatory and immune system modulating effects for the SOD-gliadin combination have also been reported, and these are discussed in this publication, with particular attention paid to the mechanism(s) behind the effects.
Reducing the production of reactive oxygen species associated with oxidative stress has many important health implications. These benefits include improved recovery after strenuous exercise, reduction of inflammation (reddening) in the skin on exposure to sunlight (UV radiation), improvement in heart health, and complications arising from diabetes. Sections in the review are dedicated to each of these areas.


The theory of free radicals in aging
Production of reactive oxygen species (ROS) is a normal process in oxygen-breathing organisms. Under normal physiological conditions a balance between these species and the body’s anti-oxidant defenses exists. (Figure 1) However, certain conditions, like smoking, pollution, exposure to sunlight (UV radiation), infection and the subsequent immune response, metabolism of sugars related to high intensity exercise, and aging, can increase the production of Reactive Oxygen Species (ROS) such as the superoxide ion (O2-) and the hydroxyl ion (OH-). This can disrupt the natural balance and lead ultimately to oxidative stress [1][2].
The detrimental health effects that can result from prolonged exposure to oxidative stress include: DNA damage that can ultimately produce cancer and atherosclerosis (hardening of the arteries) that leads to cardiovascular disease. Oxidative stress is known to significantly contribute to the process of inflammation, which underpins conditions like rheumatoid arthritis, inflammation, metabolic syndrome and diabetes, as well as to neurodegenerative diseases like Alzheimer’s [3].
SOD, the enzyme of life
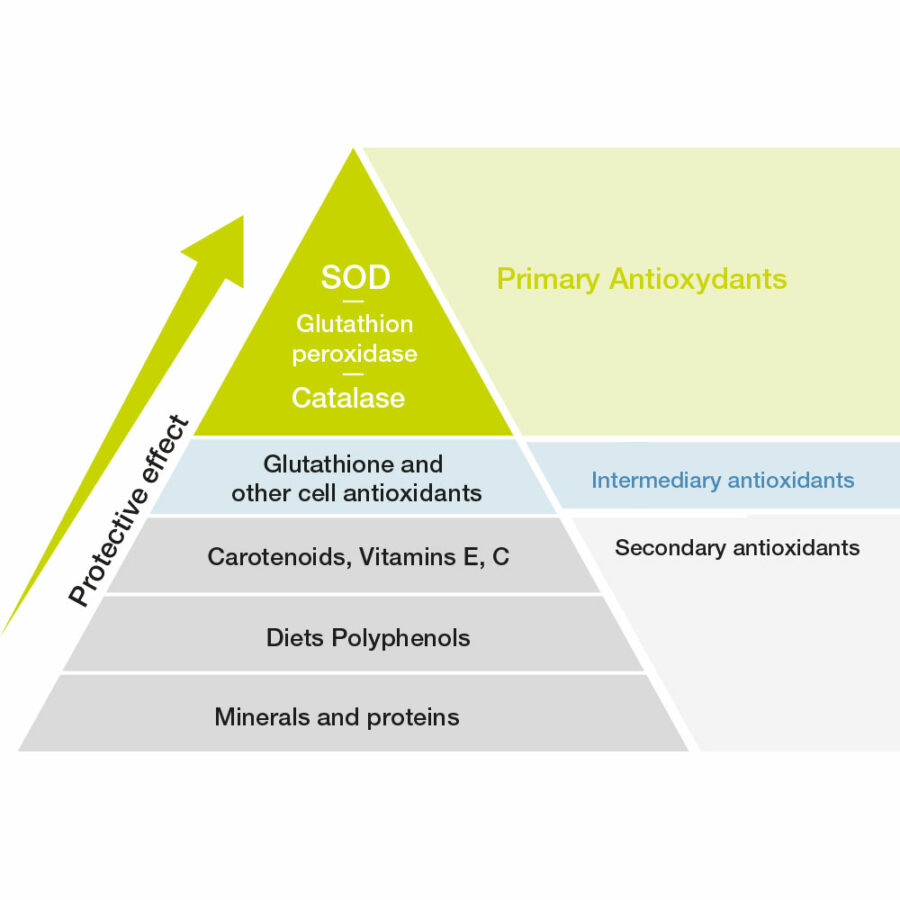
Superoxide dismutase (SOD), along with catalase and glutathione peroxidase, form the front line of the body’s antioxidant enzyme defenses [4]. The superoxide anion is the starting point of the cascade of reactions of free radical production.
SOD, dubbed the “enzyme of life” on discovery in 1968, is the first antioxidant mobilized by the cell as defense against oxidative stress. The enzyme reacts with the superoxide ion and turns it into hydrogen peroxide (H2O2). This is then catabolised by catalase and glutathione peroxidase to produce molecular oxygen (O2) and water (H2O).


An approach entirely different from conventional antioxidants
These antioxidant enzymes have a distinct advantage over the antioxidants consumed from the diet or nutritional supplements, like the vitamins A, C, and E, carotenoids, and thiols since the enzymes are biological catalysts, rapidly and repeatedly reducing reactive oxygen species without being consumed themselves. By reacting early in the process, the antioxidant enzymes can minimize the potentially-harmful oxidation of a range of biological molecules. On the other hand, a non-catalytic or stoichiometric relationship exists for most vitamins, carotenoids and thiols, meaning a defined relationship exists – one vitamin C molecule, for example, quenches just one ROS. As vitamin stores can be readily depleted under extreme free radical burden, more vitamin C must be consumed to replace that which has been lost. Moreover, in this example, as Vitamin C is involved in many other essential activities within the cell, relying on Vitamin C to quench free radicals means that Vitamin C is no longer available to perform its other essential tasks; these tasks include the production of collagen, and synthesis of certain neurotransmitters to name but two.
Biodisponibility of oral supplementation
Oral administration of SOD and the other antioxidant enzymes present in many plant extracts is, under normal conditions, not effective. During passage through the gastrointestinal pathway the enzyme is denatured (deactivated) rendering it ineffective as an antioxidant. However, studies have shown that combining a melon concentrate naturally rich in SOD with a wheat gliadin biopolymer system temporarily protects the SOD during its passage through the gastro-intestinal tract. One explanation of this efficiency was presented by Clemente et al. who showed that gliadin increases the permeability of the intestinal wall by promoting the release of a zonulin, thereby allowing the transport of the macromolecule SOD across the intestinal barrier [6].
The combination of a cantaloupe melon (Cucumis melo L.C.) concentrate naturally rich in SOD with the wheat gliadin biopolymer (Glisodin) significantly improves the delayed release of SOD as evidenced in vitro by the progressive increase of its activity in a medium mimicking the digestive conditions (Figure 3)[7].

GliSODin activates the body’s own internal antioxidant defense system, including superoxide dismutase (SOD), glutathione peroxidase and catalase: the 3 essential primary internal antioxidants
An in vivo study performed on a mouse model showed a significant increase in endogenous antioxidant levels SOD, CAT and GPx in mice supplemented with the SOD + gliadin complex, GliSODin®.After 28 days of supplementation, the SOD-gliadin group showed erythrocyte antioxidant activity four times higher than the group with SOD alone [8].
In 2006, a Japanese university published a study on the mouse model in the British Journal of Cancer highlighting the preventive role of GliSODin® in inflammation. Inducing tumour tissue in mice causes the formation of cytotoxic ROS leading to strong inflammation that can turn a benign tumour into a malignant one. The mice were divided into four groups: control (saline solution), gliadin alone, SOD alone, and GliSODin®, with two administration routes under study: oral and intraperitoneal. Results showed that only oral GliSODin® stimulated the enzymatic defense systems in mice, reducing inflammation induced by tumour formation and significantly decreasing tumour progression [5].
Overall results of in vivo research showed the bio-activity of GliSODin® taken orally and important improvements in the antioxidant status of the organism.
SOD
- GliSODin
- Placebo
- GliSODin
- Placebo
GPx
- GliSODin
- Placebo
- GliSODin
- Placebo
Catalase
- GliSODin
- Placebo
- GliSODin
- Placebo
The role of Glisodin in suppressing inflammation
An in vivo study by Vouldoukis et al.[7] submitted C57BL/6 mice groups to various supplements for 28 days: placebo, gliadin only (1 mg), SOD only (5 IU), the SOD-gliadin combination (5 mg equivalent to 5 IU of SOD, Glisodin), or heat-inactivated SOD-gliadin combination (5 mg) for 28 days and then given an intra-peritoneal INF- injection (300 IU). Peritoneal macrophages were harvested 24 hours later and challenged with IgGl/anti-IgG1 immunocomplexes to amplify the inflammatory response. Only Glisodin reduced the production of the pro-inflammatory cytokine, tumor necrosis factor-alpha (TNF-α) and promoted production of the anti-inflammatory cytokine interleukin-10 (IL-10), compared to the other treatments.
This result also showed that it is necessary to preserve the enzymatic activity of the administered-SOD to retain the anti-inflammatory effect of Glisodin® since IL-10 production was not observed when Glisodin was previously heat inactivated.
The anti-inflammatory effects of Glisodin are significant since chronic inflammation is associated with the onset and progression of many chronic diseases.
Effect of per os mice supplementation by CME, gliadin, CME/gliadin, or HI-CME/gliadin on TNF-α and IL-10 production
pg/ml
TNF-α (induces an inflammatory response)
- Placebo
- SOD only
- Gliadin
- GliSODin
- Placebo
- SOD only
- Gliadin
- GliSODin
IL-10 (induces an anti-inflammatory response)
- Placebo
- SOD only
- Gliadin
- GliSODin
- Placebo
- SOD only
- Gliadin
- GliSODin

Conclusion
Production of reactive oxygen species (ROS) is a normal process in oxygen-breathing organisms. Under normal physiological conditions a balance between these species and the body’s anti-oxidant defenses exists, but certain conditions can increase the production of ROS like the superoxide ion (O2-) and disrupt the natural balance and lead ultimately to oxidative stress.
The superoxide ion is the starting point of the cascade of reactions of free radical production. Superoxide dismutase (SOD), dubbed the “enzyme of life” on discovery in 1968, is the first antioxidant mobilized by the cell as defense against oxidative stress.
Oral delivery of the pure enzyme to boost the body’s natural antioxidant defenses has been limited by the harsh conditions experienced in the gastrointestinal (GI) passage. However, combination of SOD-rich cantaloupe melon (Cucumis melo L.C.) with the wheat gliadin biopolymer (Glisodin) can significantly and progressively increase SOD stability and delivery during passage through the GI tract, as shown by results from in vitro, in vivo and human studies.
Production of reactive oxygen species (ROS) is a normal process in oxygen-breathing organisms. Under normal physiological conditions a balance between these species and the body’s anti-oxidant defenses exists, but certain conditions can increase the production of ROS like the superoxide ion (O2-) and disrupt the natural balance and lead ultimately to oxidative stress.
The superoxide ion is the starting point of the cascade of reactions of free radical production. Superoxide dismutase (SOD), dubbed the “enzyme of life” on discovery in 1968, is the first antioxidant mobilized by the cell as defense against oxidative stress.
Oral delivery of the pure enzyme to boost the body’s natural antioxidant defenses has been limited by the harsh conditions experienced in the gastrointestinal (GI) passage. However, combination of SOD-rich cantaloupe melon (Cucumis melo L.C.) with the wheat gliadin biopolymer (Glisodin) can significantly and progressively increase SOD stability and delivery during passage through the GI tract, as shown by results from in vitro, in vivo and human studies.

References
(5) Di Massimo C., Scarpelli P., Di Lorenzo N., Caimi G. di Orio F., Ciancarelli M.G., “Impaired plasma nitric oxide availability and extracellular superoxide dismutase activity in healthy humans with advancing age” Life Sciences. 2006, Volume 78, Pages 1163-1167
(16) Shawn M. Arent, PhD, “Nutritional Supplementation (Glisodin® Containing Resurgex® Formula) In Male College Football Players: Effects On Strength, Body Composition And Oxidative Stress,” Human Performance Lab, Rutgers University, New Brunswick, NJ. 2007.
(19) DermExpert Trial, “Evaluation Of Glisodin’s Effect On Erythema Induced By UV Radiations,” Intermediate Reports, February 2006